Wearable Sensing and Imaging Technologies
Thrust Leads
Goal: Thrust 3 will develop wearable devices for blood pressure, heart rate and heart rate variability monitoring and readers for the implantable bar-code sensors for point-of-care (POC) monitoring of cardiovascular disease and diabetes biomarkers. In addition, T3 is developing realistic computational models in support of all thrusts that account for patient-specific anatomy and physiology including skin tone and elevated Body Mass Index.
Project 3.1: Enabling wearable technologies for individuals experiencing disability within the target PATHS-UP communities
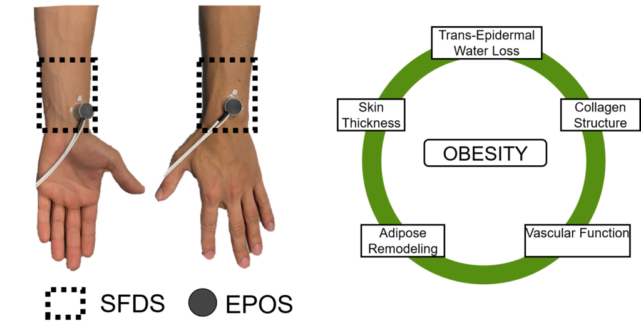
Obesity afflicts a large portion of the PATHS-UP target population. Through computational work, we have demonstrated that the combination of obesity and elevated skin tone has dramatic effects on optical signals such as the ones found in photoplethysmography (PPG). Our goal in this project is to focus on the unique challenges of the obese population within and refine the PATHS-UP wearable technologies through a combination of computational modeling and human studies aimed at characterizing the skin of the obese.
THRUST 3.1 RESEARCH HIGHLIGHTS
Biomedical Optics Express (2021)
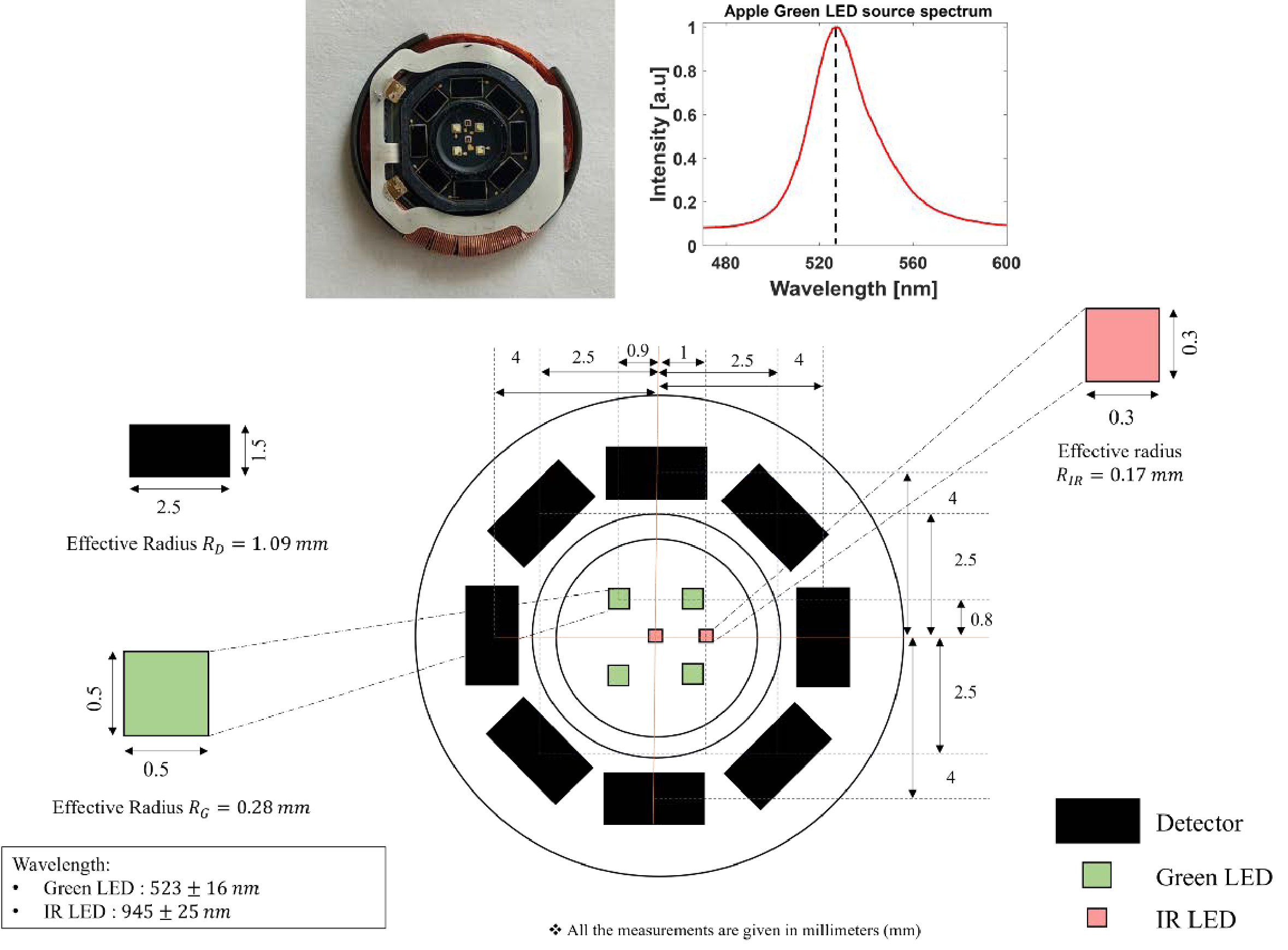
Ajmal, Tananant Boonya-Ananta, Andres J. Rodriguez, V. N. Du Le, and Jessica C. Ramella-Roman
Biosensors (2021)
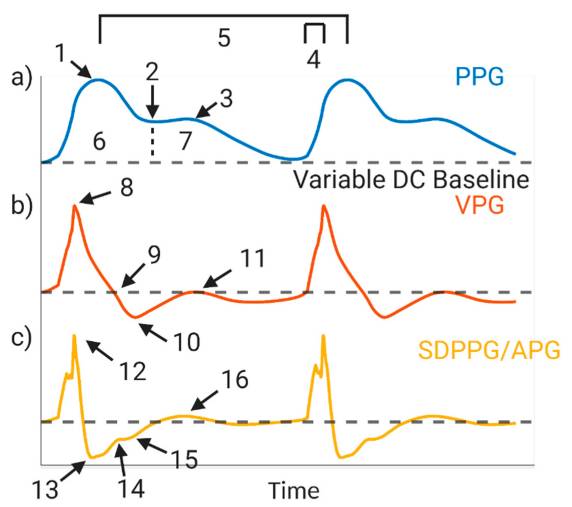
Jesse Fine, Kimberly L. Branan, Andres J. Rodriguez, Tananant Boonya-ananta, Ajmal, Jessica C. Ramella-Roman, Michael J. McShane and Gerard L. Coté
Project 3.2: Multi-modal physiological wearable sensing platform
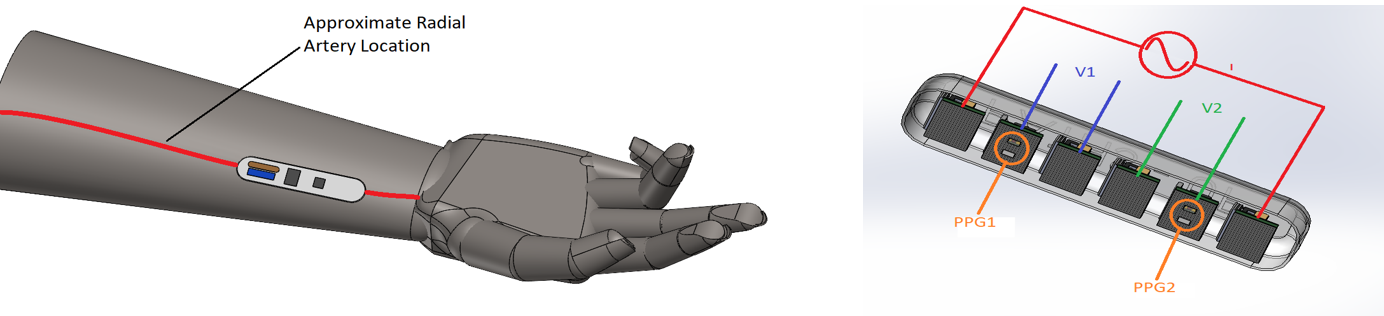
This project aims to develop a multimodal physiological sensing wearable device that will be placed along the radial artery and will be used to measure heart rate, heart rate variability, and cuffless blood pressure.
THRUST 3.2 RESEARCH HIGHLIGHTS
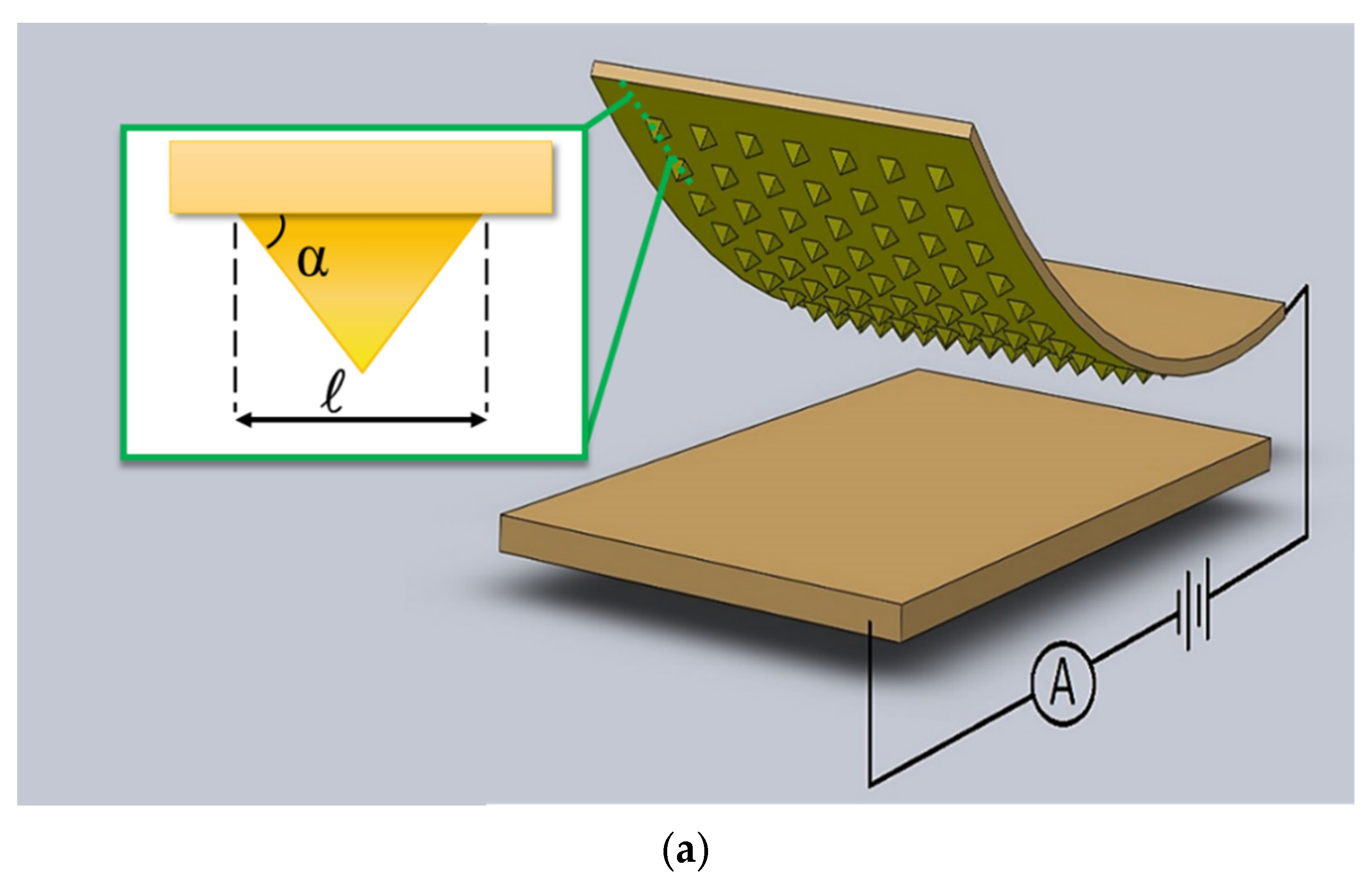
Micromachines (2022)
Borzooye Jafarizadeh, Azmal Huda Chowdhury, Iman Khakpour, Nezih Pala and Chunlei Wang
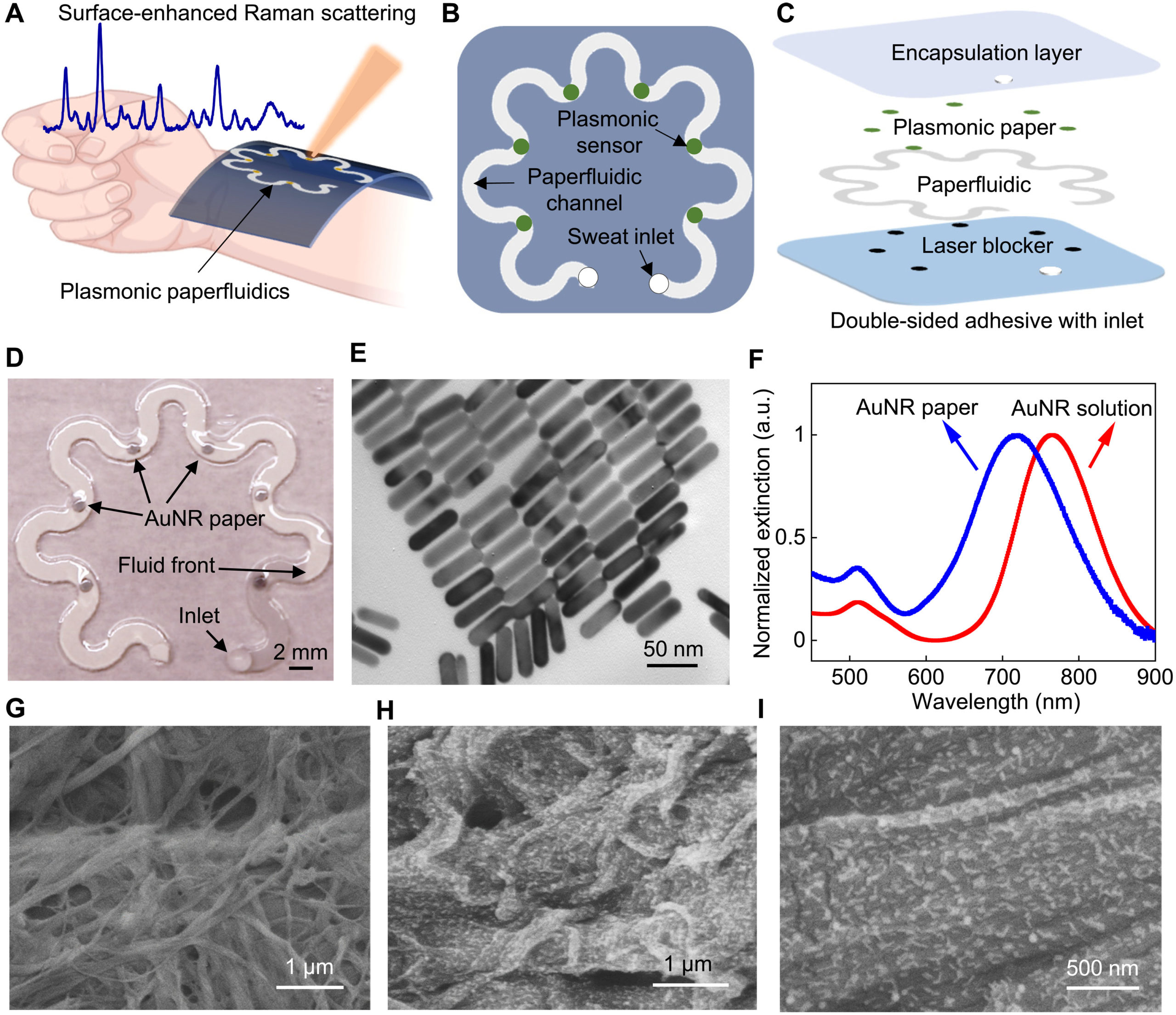
Science Advances (2022)
Umesha Mogera, Heng Guo, Myeong Namkoong, Md Saifur Rahman, Tan Nguyen, Limei Tian
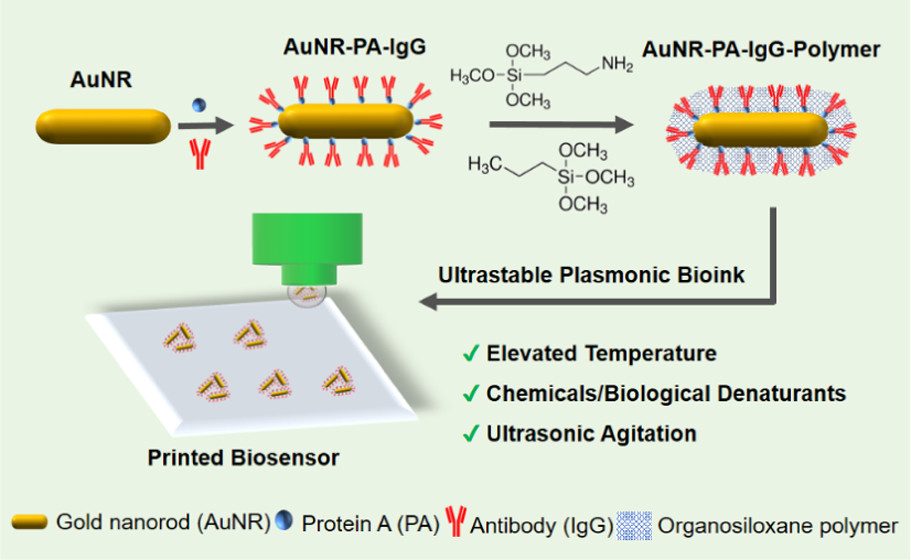
ACS Applied Materials & Interfaces (2021)
Ze Yin, Heng Guo, Yixuan Li, Joshua Chiu, and Limei Tian
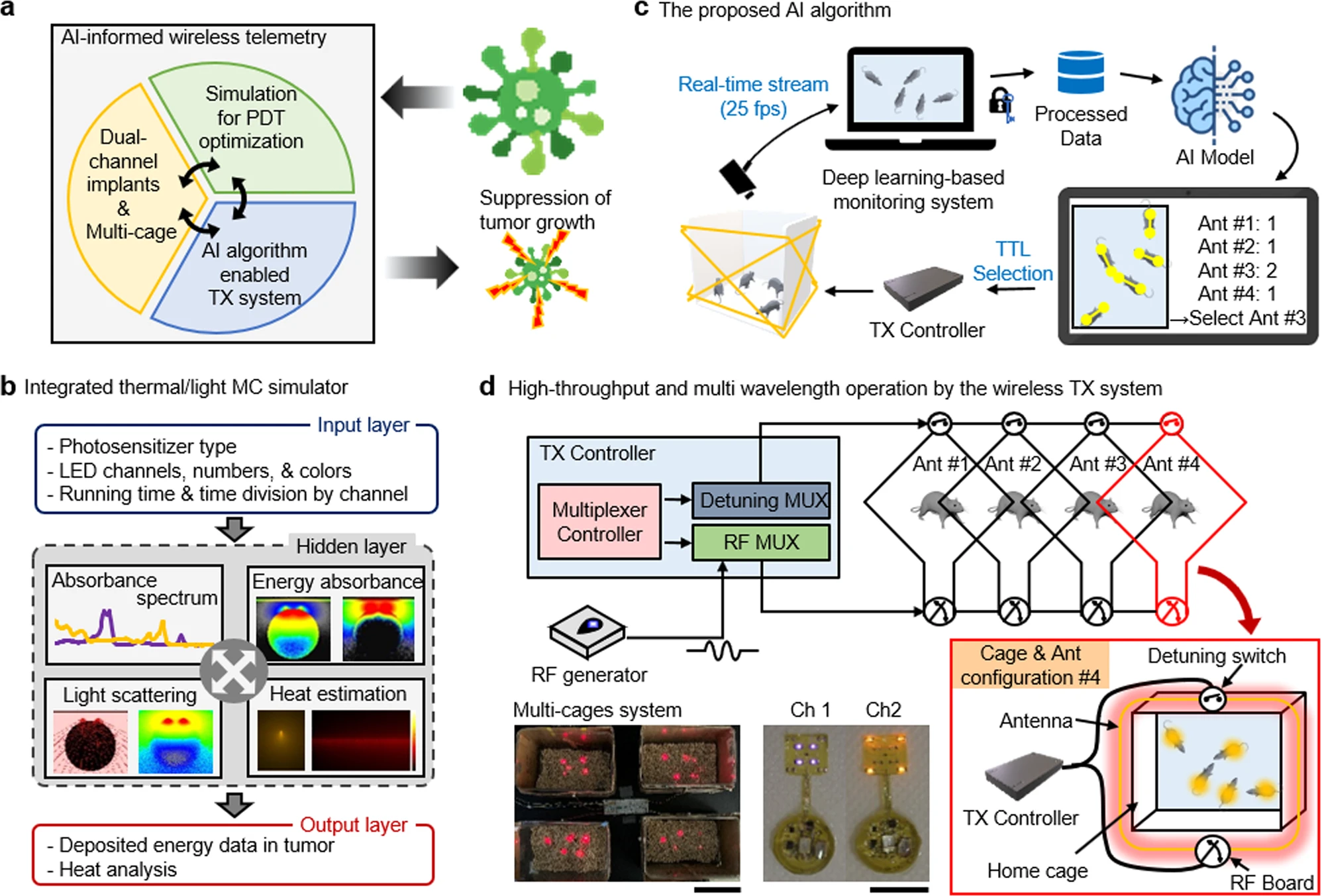
Nature Communications (2022
Woo Seok Kim, M. Ibrahim Khot, Hyun-Myung Woo, Sungcheol Hong, Dong-Hyun Baek, Thomas Maisey, Brandon Daniels, P. Louise Coletta, Byung-Jun Yoon, David G. Jayne & Sung Il Park
Project 3.3: Wearable high resolution optical imagers
This project focuses on designing and building a wearable imager with integrated illumination for measuring vital information, such as glucose and oxygen content in blood, through the skin tissue. The vital information will be measured via the response of an implanted barcode that is being developed in Thrust 1. The wearable imager is designed to image the barcode through tissue scattering with sufficient spatial resolution and will have controlled illumination to activate and record the phosphorescence lifetime response of the barcode.
Jessica Ramella-Roman
Ashok Veeraraghavan
Aydogan Ozcan
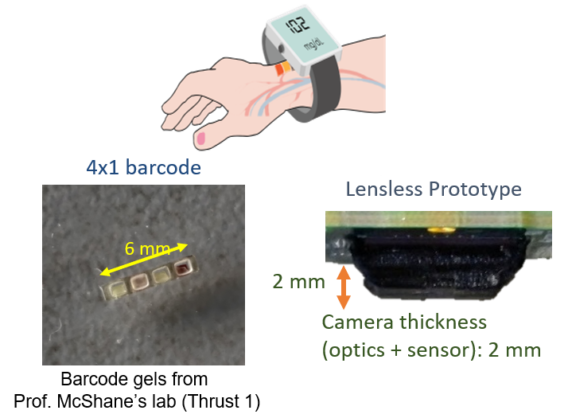
THRUST 3.3 RESEARCH HIGHLIGHTS
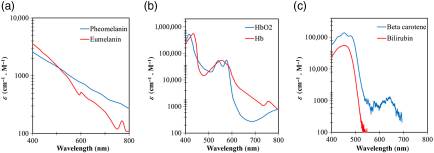
Journal of Biomedical Optics (2022)
Andres J Rodriguez, Mel Tananant Boonya-Ananta, Mariacarla Gonzalez, Vinh Nguyen Du Le, Jesse Fine, Cristina Palacios, Mike J McShane, Gerard L Coté, Jessica C Ramella-Roman
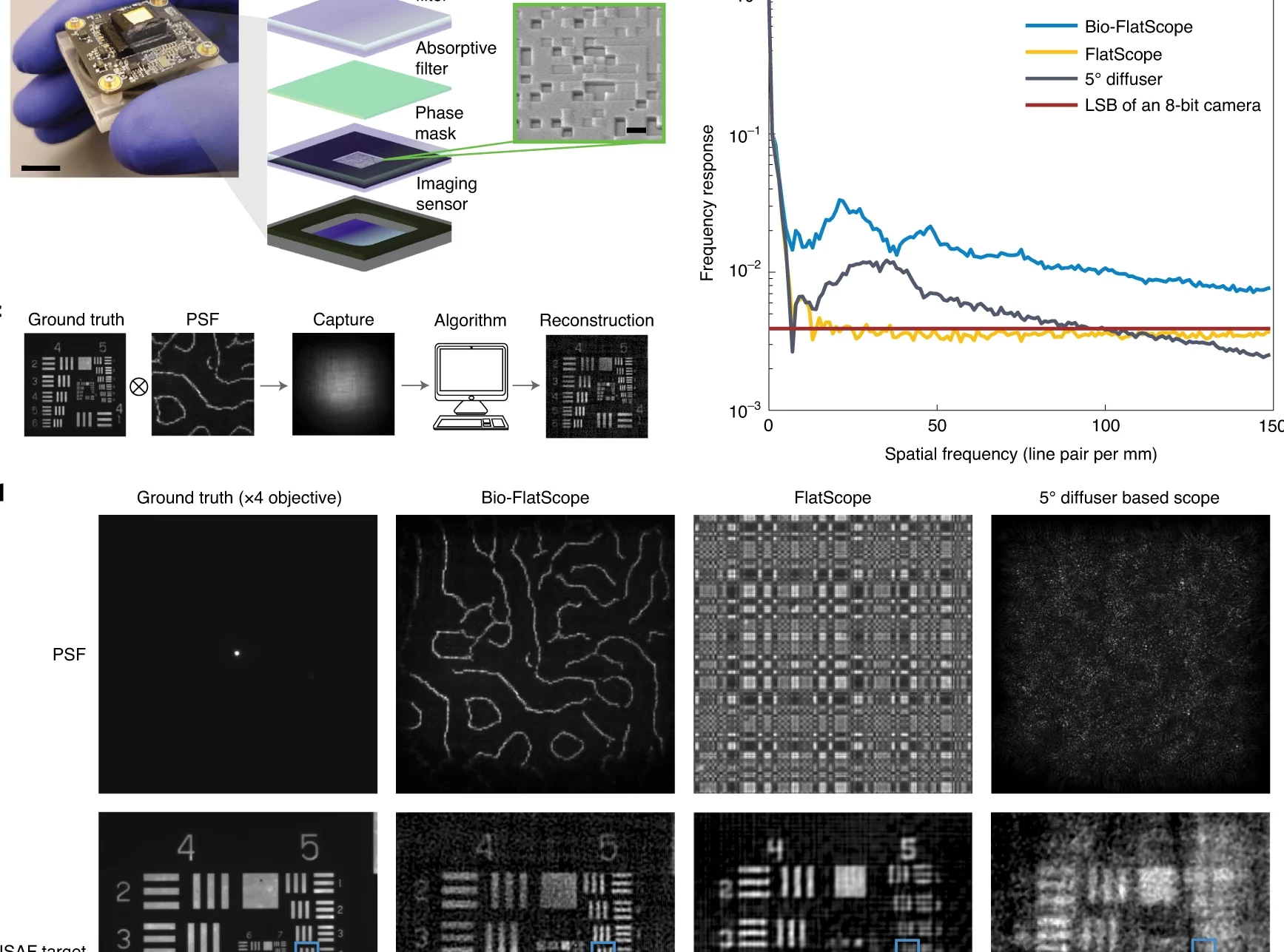
Nature Biomedical Engineering (2022)
Jesse K. Adams, Dong Yan, Jimin Wu, Vivek Boominathan, Sibo Gao, Alex V. Rodriguez, Soonyoung Kim, Jennifer Carns, Rebecca Richards-Kortum, Caleb Kemere, Ashok Veeraraghavan & Jacob T. Robinson
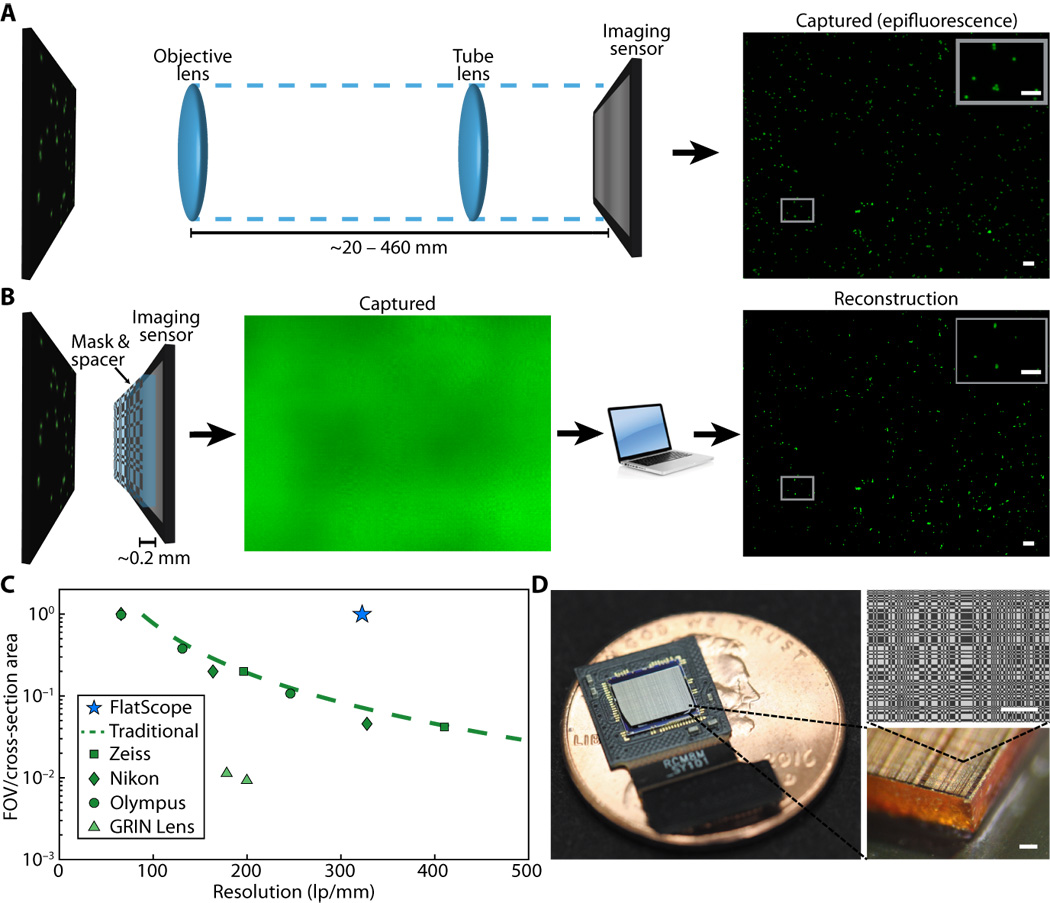
Science Advances (2017)
Jesse K Adams, Vivek Boominathan, Benjamin W Avants, Daniel G Vercosa, Fan Ye, Richard G Baraniuk, Jacob T Robinson, Ashok Veeraraghavan







